Bacteria in Alzheimer's Disease
For decades, Alzheimer's disease (AD) research has been dominated by the amyloid cascade hypothesis, focusing on the accumulation of amyloid plaques in the brain. However, recent studies have brought to light a new paradigm that could reshape our understanding of this devastating condition. More recent research utilizing advanced sequencing technology has revealed compelling evidence of a bacterial component in the development of Alzheimer's disease. A new study published this week also suggests a connection between AD and a pathogenic microbiome in the brain, which may result from a compromised blood-brain barrier (BBB).
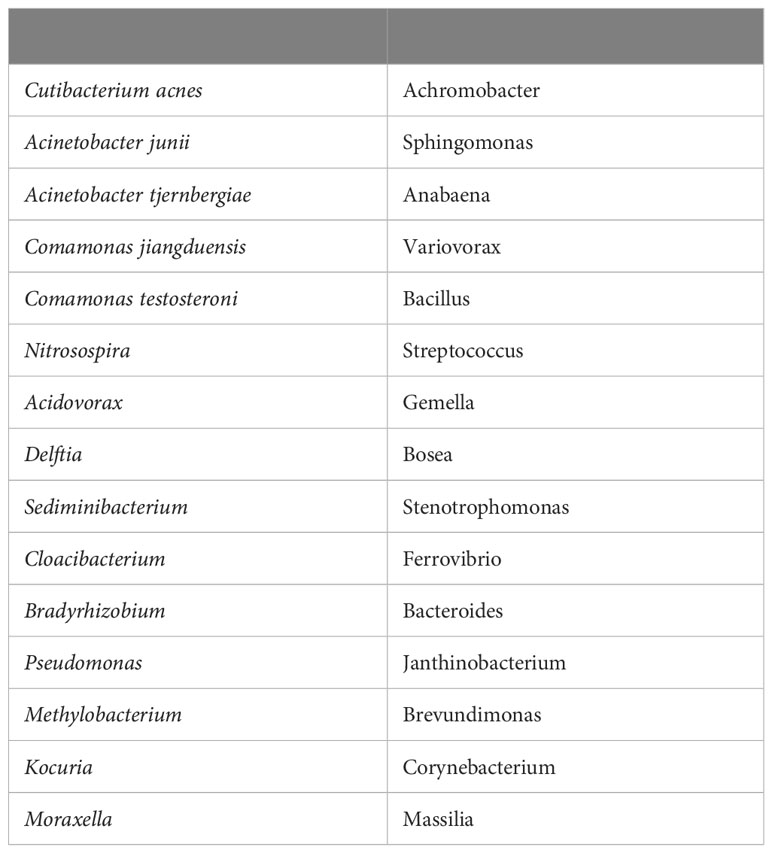
The study analyzed postmortem brain samples from 32 individuals, comprising 16 AD patients and 16 age-matched control subjects. A total of 130 samples were collected from various brain regions. Researchers employed full-length 16S rRNA gene amplification with Pacific Biosciences sequencing technology to identify bacteria within these samples. Notably, they detected bacteria in both AD and control brains, with Cutibacterium acnes (formerly Propionibacterium acnes) and two species from the Acinetobacter and Comamonas genera being the principal bacteria identified. The table below is Table 1 from the article, listing top 30 genera/species by prevalence in order top to bottom, left to right.
The study's findings revealed a complex polymicrobial dynamic associated with AD-related pathogenicity. Interestingly, the abundance of C. acnes in the brain seemed to follow a pattern of rise and fall, with pathogenicity occurring at an off-peak abundance level. Importantly, this pathogenicity was linked to the presence of at least one other bacterium from a set of genera, including Methylobacterium, Bacillus, Caulobacter, Delftia, and Variovorax. This intricate interaction between bacteria suggests a multifaceted role in AD development.
This study is suggesting a leaky blood-brain barrier or lymphatic network in AD patients. This potential breach in the protective barrier could enable bacteria, viruses, fungi, or other pathogens to enter the brain, potentially contributing to disease progression. Healthy brain needs healthy vascular system for its normal functioning.
These findings align with the emerging "pathogen hypothesis" and "antimicrobial protection hypothesis," which propose that various microorganisms, including bacteria and viruses, play a role in AD pathogenesis. This paradigm shift is supported by the detection of diverse pathogens, such as Herpes simplex virus type 1 (HSV1), Borrelia burgdorferi (Lyme disease agent), Treponema spp., Chlamydia pneumoniae, Porphyromonas gingivalis, and Cutibacterium acnes in postmortem AD brains.
BBB breakdown is proposed to happen in Alzheimer disease, Parkinson disease, Huntington disease, amyotrophic lateral sclerosis, multiple sclerosis, HIV-1-associated dementia and chronic traumatic encephalopathy. Breakdown of the BBB enables toxic blood-derived molecules, cells and microbial agents to enter the brain. It can happen because of the damage to blood vessels, chronic conditions such as diabetes (impaired glucose transport), pericyte and endothelial cell degeneration, autoimmune diseases and infections inflaming brain's blood vessels.
As research in this area continues to evolve, it may offer promising avenues for understanding, preventing, and treating these devastating neurological disorders.
REFERENCES
Moné Y, Earl JP, Król JE, Ahmed A, Sen B, Ehrlich GD, LapIdes JR. Evidence Supportive of a Bacterial Component in the Etiology for Alzheimer's Disease and for a Temporal-Spatial Development of a Pathogenic Microbiome in the Brain. Frontiers in Cellular and Infection Microbiology.;13:1123228. https://doi.org/10.3389/fcimb.2023.1123228
Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018 Mar;14(3):133-150. doi: 10.1038/nrneurol.2017.188. Epub 2018 Jan 29. PMID: 29377008; PMCID: PMC5829048.



Comments
Post a Comment